The term "colloid" from the Greek words kolla, meaning "glue" and eidos, meaning "like" was first used in 1861 by Thomas Graham to classify mixtures such as starch in water and gelatin.
Many colloidal particles are aggregates of hundreds or thousands of molecules, but others (such as proteins and polymer molecules) consist of a single extremely large molecule.
Proteins and synthetic polymer molecules that form colloids can have molecular masses ranging from a few thousand to many millions of atomic mass units.
ACTION
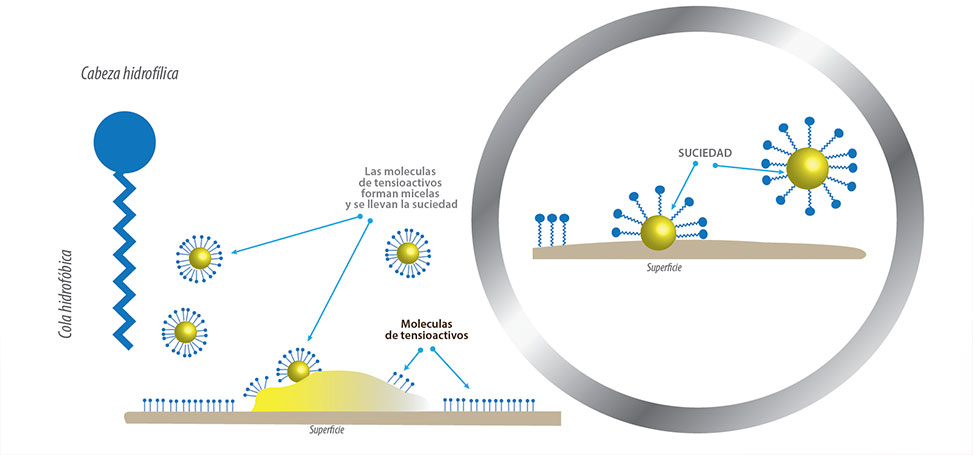
The cleaning action of soaps and detergents can be explained in terms of the structures of the molecules involved.
The hydrocarbon (nonpolar) end of a soap or detergent molecule dissolves or is attracted to nonpolar substances such as oil, grease, or dirt particles.
The ionic end is attracted to water (polar). As a result, soap or detergent molecules orient themselves at the interface between dirt particles and water, thus acting as a kind of bridge between two different types of matter, nonpolar and polar.
Molecules like this are called amphiphilic as they have a hydrophobic part and a hydrophilic part.
As a consequence, the dirt particles are suspended as colloidal particles and are easily removed.